In the last two editions of The Chloroquine Wars, we showed that,
RCTs are not guaranteed to succeed in demonstrating a superior result,
Historically, RCTs demonstrate the same results as observational studies, and
Now we delve into what the RCTs testing the efficacy of hydroxychloroquine (HCQ) demonstrate about their efficacy in treating SARS-CoV-2/COVID-19 patients. We begin with the big picture of all 29 such RCTs, summarized by the COVID Analysis team at hcqmeta.com.
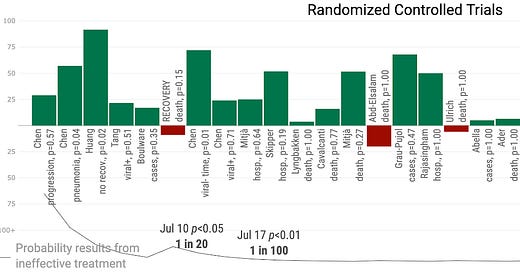
First, let us analyze the totality of results without sorting out which studies we consider of superior quality---a task we save for future articles.
While not all the results were well-powered to achieve statistically significant results on their own, a meta-analysis of effects shows that the likelihood of achieving these results at random is less than one in a thousand (this is the correct meaning of p < 0.001). On face, this meta-analysis seems demonstratively to indicate HCQ helps sufferers of COVID-19. And, as we should predict, the distribution of results in these RCTs looks a great deal like the results of other studies, including many observational studies.
This similarity suggests that we look at the entire body of research to get a true sense of the likelihood that HCQ effectively treats the infection or disease:
Most people do not realize that there are well over 200 studies to date on HCQ efficacy in treating COVID-19 patients. This is now one of the most well-studied treatments in the history of medicine, and the evidence is overwhelming. The chance that these results could occur at random is approximately 1 in 208 quadrillion. That is, p = 0.000000000000048. Having read a few thousand research papers, I have never before seen a p-value this low in the testing of medicine. I put the question to numerous experienced researchers, with none able to find such a result. (If you happen upon one, please comment with a link for the sheer trivia value.)
Next, we would be remiss if we did not point out that the RCT headed by Grau-Pujol showed no serious adverse events among healthy HCWs using a low dose of HCQ as pre-exposure prophylaxis. This and nearly all of these other studies, not to mention the long safety history of HCQ, properly lay to rest the notion that HCQ is dangerous for COVID-19 patients---at least at the doses studied in around 98% of these studies. And, as we discuss in future articles, some of the least favorable studies, including those conducted by the WHO, used doses far higher than those used by any other doctors around the world, and for which there is evidence of harm.
Finally, let us consider the common sense notion that the best use of HCQ is as an early treatment medication. The reason that HCQ and CQ were the first medicines off the shelf to be tested was due to previous studies showing their in vitro antiviral action against other coronaviruses. It makes sense to focus in on those studies testing HCQ prior to significant disease advancement (no later than the fifth day of symptoms).
It is hard to fathom looking at these results and not thinking they make an overwhelming case for HCQ efficacy against SARS-CoV-2 and COVID-19. If there are any studies missing from this analysis, please comment. Thank you.







Very much look forward to your book on CQ/HCQ.