Why is the U.S. Government Purchasing a Drug that Treats Radiation Blood Poisoning and a Common Vaccine Side Effect?
The Healthcare Wars, Part 2
My friend Stacia (who took part in our last weekly Roundtable), who is the founder of 2nd Look Media, sent me a particularly unusual article a little while ago. It's straight from the newsroom at Health and Human Services (HHS):
With the conflict raging in Ukraine, many people are discussing the potential for nuclear conflict. But is that what this is really all about?
Before I jump to the interesting parts of the article, I'd like to mention that I personally doubt that we will see nuclear weapons used directly between major world powers. The result is suicide. However, we should fear the use of weapons of mass destruction anywhere proxy wars are wages, and we should worry about the potential for a disguised state actor or even independent actor (or an intelligence agency pretending to be one of those) using ugly weapons anywhere on Earth, including at home.
But is that what this is really all about?
I ask the question because of the dual uses of the drug. First,
As part of long-standing, ongoing efforts to be better prepared to save lives following radiological and nuclear emergencies, the U.S. Department of Health and Human Services is purchasing a supply of the drug Nplate from Amgen USA Inc; Nplate is approved to treat blood cell injuries that accompany acute radiation syndrome in adult and pediatric patients (ARS).
Amgen, based in Thousands Oaks, California, developed Nplate for ARS with support from the Biomedical Advanced Research and Development Authority (BARDA), part of the HHS Administration for Strategic Preparedness and Response (ASPR), as well as the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health.
BARDA is using its authority provided under the 2004 Project Bioshield Act and $290 million in Project BioShield designated funding to purchase this supply of the drug. Amgen will maintain this supply in vendor-managed inventory. This approach decreases life-cycle management costs for taxpayers because doses that near expiration can be rotated into the commercial market for rapid use prior to expiry and new doses can be added to the government supply.
ARS, also known as radiation sickness, occurs when a person’s entire body is exposed to a high dose of penetrating radiation, reaching internal organs in a matter of seconds. Symptoms of ARS injuries include impaired blood clotting as a result of low platelet counts, which can lead to uncontrolled and life-threatening bleeding.
So far this just sounds like a (high dollar) project to purchase a drug that can be delivered to large numbers of people in the event of a nuclear blast. But here is the strange part:
Nplate is also approved for adult and pediatric patients with immune thrombocytopenia, a blood disorder resulting in low platelet counts. Repurposing drugs for acute radiation syndrome that also are approved for a commercial indication helps to sustain availability of the product and improves healthcare provider familiarity with the drug.
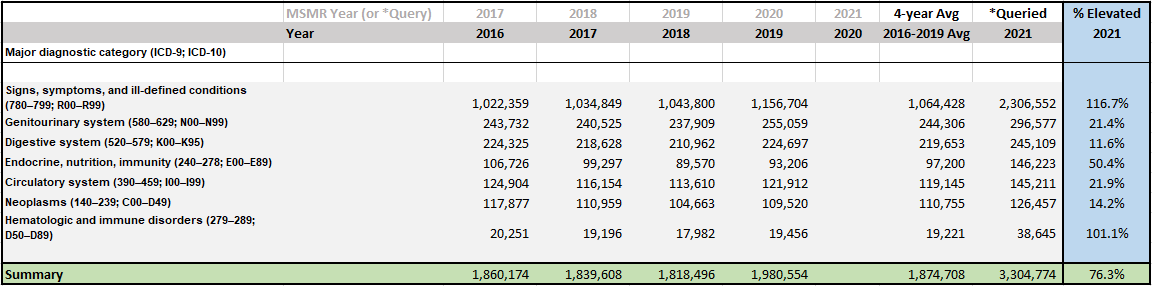
If you're not yet aware, immune thrombocytopenia is one of the uglier side effects we've seen since the start of the experimental COVID-19 mass transfection campaign. As I noted during the VSRF conversation yesterday, and in a prior article, Hematologic and immune disorders are as a group the largest form of illness increase in the military health database. I've had confirmation of this observation from numerous sources and shared physician experience.
What does this all mean? I don't know. It could be a coincidence that Amgen is getting paid hundreds of millions to deliver this dual-use drug. It could also be that the drug gets administered to insiders, while worries over nuclear fallout and radiation sickness are simply a way to justify the expense.
What do you think?
Addendum: One of the doctors I know says this is fishy because the drug doesn’t cure radiation poisoning—it just stops hemorrhaging.
Addendum: Hat tip to a couple of readers who pointed out that Coffee and COVID has a more thorough article the topic:






JC over at coffeeandcovid.com reported on this this morning. He says the drug's website doesn't mention anything about treating radiation sickness.
Of course the feds will continue to funnel tax money to Big Pharma. But those behind the scenes never do anything for just one reason. I guess we'll see.
Have you read Jeff Childers's fascinating theory (https://www.coffeeandcovid.com/p/c-and-c-news-friday-october-7-2022) about this? He's thinking along the same lines. I'm also wondering about 5G in relation to this, but that is a rabbit-hole I have not had time to hop down and am unversed on, although this article (https://anamihalceamdphd.substack.com/p/fifth-generation-5g-directed-energy) may be of interest.