Nonsensical, Procedurally Invalid Vaccine Trial Results
The Vaccine Wars Part LII
"The greatest obstacle to discovery is not ignorance—it is the illusion of knowledge." Daniel Boorstin
If you are interested in how vaccine trials can be manipulated, you're going to want to read this article through completely.
Earlier this week on Monday I had a great conversation with Nutrition Scientist Chris Masterjohn. Our conversations have been extremely important for me in a way that I will explain later in this article. I believe that our observations led to something that vaccine experts likely knew from the start: the trials were designed so that the results are functionally meaningless, but serve as procedural illusions.
Edit: Since YouTube saw fit to censor this discussion, viewers must go here to watch it on Rumble.
I invited him to a live conversation due to his excitement over strange observations about adverse events in vaccine trial reports such as coughing and dyspnea (trouble breathing). In at least one of the trial reports, dyspnea was broken up into subcategories in a way that looks like a sneaky justification for leaving the data off the top level trial report. Perhaps more importantly, setting aside these side effects is supportive evidence that the vaccine trials relied on statistical illusions to make efficacy claims. Chris goes into detail about these problems in our conversation, but our discussions led to a much more serious observation about how Operation "skip important steps" Warp Speed gave the vaccine manufacturers the green light to run completely invalid trials.
Zero Efficacy or More Disease?
Multiple times over the past few months, Chris and I have been in email exchanges about a suspicion that the vaccines change the sensitivity/specificity calculations for PCR (or other) tests, thus resulting in massive changes in false negative rates in particular. This article is from before I'd met Chris, but he mentioned it during our discussion earlier this week:
I (strongly) recommend reading the whole article, but here is one key piece:
The “Hospitalization Paradox” is my name for the following seemingly paradoxical observations:
The COVID vaccines strongly reduce the chance of a positive PCR nasal swab among anyone suffering from COVID-like illness.
By contrast, they have little to no effect in reducing total COVID-like illness of any severity, nor in reducing hospitalization for it.
During the first two to three months after vaccination while the vaccines maintain high efficacy against a positive test, they do not have any apparent effect on hospitalization, even among those who test positive.
As the vaccines begin declining in efficacy against testing positive, they still have no meaningful impact on hospitalization for total COVID-like illness among the vaccinated population at large.
And here is the paradoxical part: during this waning phase, specifically among the growing number of vaccinated people who do test positive, they become associated with a lower risk of hospitalization.
Let me summarize the point:
Reducing testing positivity is not the same thing as reducing disease.
Now, there are going to be people who experience cognitive dissonance over this point. Perhaps many people assume, without checking the literature, that vaccines would not confound tests for viral or antibody presence. But those people are wrong.
Vaccines can lead to viral mutations that confound PCR results (Davidson et al, 2009), and there is substantial evidence of vaccine-driven viral variation (here and here) that resulted in the use of the S-gene primer to fall out in the PCR testing (Brown et al, 2021; Lewnard et al, 2021) over and over during the pandemic because the [likely unnatural] spike protein is subject to a higher mutation rate than the rest of the SARS-CoV-2 viral genome (Laha et al, 2020).
Potential for vaccination confounding has been noted for detection of viruses due to prior infection (Foppa et al, 2019) even for a virus (influenza) that, like coronaviruses, changes in variation between seasons and years.
Researchers (Follmann et al, 2022) have noted a 0.43 ratio of the frequency of anti-nucleocapsid antibodies between the vaccine and placebo arms (quote: "However, it is unclear if pre-infection vaccination can affect the seroconversion rate and the sensitivity of antibody testing."). And a certain censored mouse found a 75 to 160 count (ratio 0.47) of anti-N ABs in the Pfizer trial data. If vaccines confound anti-N antibody testing as a viral detection tool, why should we assume (without validation) that they cannot confound the PCR tests used?
We have no reason to assume that vaccines do not confound the tests used, and we should demand proof that they do not before giving the otherwise highly suspect vaccine trials any level of credibility.
But the vaccine manufacturers and their circle have gamed the system, substituting positive/negative testing as a false proxy output in place of disease, as was traditionally defined by symptoms and biological damage.
Some symptom summaries may be even worse in the vaccine arms, but the larger point is that the primary COVID symptoms are in proportion suggestive of the Zero Vaccine Efficacy Hypothesis.
How the Magic Trick Works
If you haven't read this article of mine from October, it's a short, but important read:
The World Health Organization (WHO) appears to have successfully led a shift in both the definition of disease, and the convention for naming disease by etiology instead of the classic focus on symptomatic presentation. The insertion of etiology into the categorization is the reason we don't have "Type 2 COVID-19" which would be COVID-19 induced by vaccines. To most people, this shift is innocuous, or just assumed as a necessary or whimsical reorganization of definitions (note that there was never a mass reorganization of past terminology). But what appears to be merely rhetorical winds up becoming the lynchpin for a grand illusion:
An agent ("treatment") that masks an etiology (cause) of a disease without preventing the totality of symptoms is "effective" [by definition].
Voila!
To pull it off, you need each of the following:
An accepted disease definition of exclusive etiology so that testing for the presence of the etiological source (the virus) serves as a "logical" proxy for the test of disease [as would be classically measured by symptoms].
A test and vaccine combination that is confounding, and
for which the test for confounding interference is never [publicly] performed.
Methods for hiding the fact that the summary symptomatic presentation in the two arms is roughly the same.
The only piece of this that we do not know 100% for certain is the second piece. But it's very possible that this was the reason why the mysterious two month delay for a PCR test occurred. After all, we've never been given a truly reasonable explanation for why the CDC and FDA fumbled that ball (since they're so competent at everything else). And we do have the hospital evidence of COVID+COVID-like-illness occurring in roughly equal proportions between the vaccinated and unvaccinated, not to mention all the supporting evidence of the Zero Efficacy Hypothesis.
But understand, even if the hypothetical second piece (the conspiratorial piece) is false, the simple fact remains:
The trials tested for whether the quasi-vaccines reduced positive tests, not for whether they reduced either infection or disease.
Perhaps somebody has realized this before now. In fact, I very much hope I'm not the first to observe the magic trick, but it's very possible that the global organizations controlling the vaccine industry paid, murdered, or struck fear into almost every single human being who understood vaccine trials well enough to pick through the logic completely (not that my picking was anything like complete before this all occurred to me).
This is enough to demonstrate the rubber-stamping of the trials. And we are a hair's breadth from demonstrating their total invalidity.
A Final Note on Validation of Non-Confounding Vaccination
I leave the door open to being wrong. Maybe the tests used were demonstrated to have highly similar sensitivity and specificity for the vaccinated and unvaccinated alike. I currently doubt it. But the burden of proof should be on the people screaming "well-run clinical trial" and "we saved millions of lives" in the face of so many worrying signals of increased mortality (and illnesses likely to cause more excess mortality going forward), and seemingly no warm and fuzzy national case studies outside of the handful of Western-sphere nations that control the pro-pharma, pro-vaccine paradigm.
As part of my logical exploration presented in this article, I made phone calls to several biologists including J.J. Couey and Robert Malone. They both agreed that trial validity hinges on validation that the vaccines do not confound the PCR tests used to compute efficacy. I learned from Malone that such validation is ordinarily part of the long vaccine testing process (because of course, it must be in order to reach a valid and meaningful result). He then encouraged me to track down research on the PCR tests used, and to be certain that no such validity testing took place.
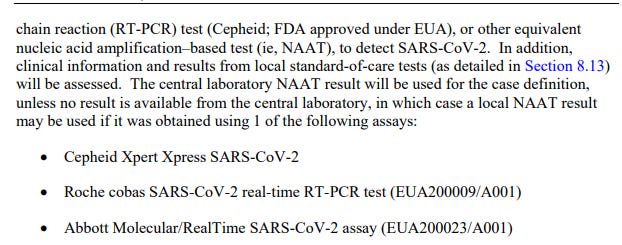
In Section 8.1 (page 51) of Pfizer's Clinical Protocol Template, we see that only nasal swabbing is tested. If testing validity were a concern, I would expect to see something like a proportion of the pool being tested through lung lavage, anal swab, or possibly other methods. Three different assays were available for the process.
Of course, the EUA's for these PCR assays were handed out prior to vaccine production, so the EUAs do not signify testing for lack of vaccine confounding.
Cepheid is a Bay Area diagnostics corporation with DARPA ties and supports researchers who specifically study SARS-CoV-2 infection-elicited expressions (Gentles et al, 2022), and is also accused of pandemic profiteering, but let's ignore these observations of tertiary importance for the moment. The most important observation we can make is that the company admitted that their test, which looks for an N2 target region (ahem) could have reduced sensitivity to SARS-CoV-2 variants.
Wojewoda noted that test manufacturers developed their tests based on genes that would be less likely to change over time: "The N1, N2, and E regions didn't seem to have as much of a selection advantage," she said.
Considering that this is the primary test in the Pfizer protocol, I'd say things don't look good for testing validity, but I'm going to call on readers to help. I do not see evidence of testing for the lack of vaccine confounding for these tests. Let me know if you find it, or know where it's hiding. For the moment, I have substantial doubts of its existence.






I am reliably told that it is in fact the unvaccinated, not the vaccines, that lead to increased rates of mutation and variability.
I am uncertain as to what mechanism causes these evolutionary pressures in a normal, unaltered host immune system, but I do know that the unvaccinated are bad, so it is logical that they would incubate bad things in the manner of a witch's third nipple nursing a homunculus.
This leads me to a complete loss to explain the above discussion, so I am choosing to ignore it, out of an abundance of caution.
My trip (with my sick dog) to the veterinarian confirmed the PCR fraud.
In veterinary medicine, PCR is used to dismiss a targeted viruses as the cause of symptoms. Not to confirm it as the cause. If you test for the Bordetella virus, and the PCR shows negative, you look to other causes for the symptoms seen. If the PCR shows positive, it you assume nothing and keep looking. The PCR is understood as prone to false positives. Covid mandates though made the false positive problem exponentially worse.
Before Covid, the labs provided quantitative PCR. That is, they told the vet at what cycle threshold the test turned positive. The Vet could then use their judgement and experience in accessing the validity of the result. After Covid mandates, our government made it unlawful for labs to provide quantitative data and only allowed qualitative (True or False) results. The lab my vet used had a big disclaimer on their website apologizing for the change and asking customer for their understanding. This lab performed PCR tests for both humans and animals.